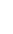It’s All Around Us
As the nucleus of a uranium atom (or any other unstable element) attempts to become more stable, it releases energy in the form of subatomic particles and/or electromagnetic waves. This process is called radioactive decay or transformation. The particles which are emitted are alpha (α) particles (consisting of two protons and two neutrons), beta (β) particles (electrons), and neutrons. Electromagnetic waves called gamma (γ) rays are similar to radio waves or light but are typically emitted with much higher energies.
These emitted particles and waves are known collectively as radiation.
There are also two forms of radiation: non-ionising and ionising. Non-ionising radiation makes up our visible light, radio and television signals, emanates from our computer screens and heats our food in microwave ovens.
When radiation has enough energy to remove the electrons of atoms with which it interacts (changing the composition of the original atom itself) it becomes charged, or ionized, and is therefore called ionizing radiation. Ionizing radiation occurs in several forms - as alpha particles, beta particles or neutrons, or in the form of electromagnetic radiation (gamma rays and X-rays).
Alpha particles consist of two protons and two neutrons, identical to the nucleus of a helium atom. A sheet of paper or a person's surface layer of skin will stop them. Alpha particles are only considered hazardous to a person's health if they are ingested or inhaled and thus come into contact with sensitive cells such as in the lungs, liver and bones.
A natural source of alpha particles is radon gas, found almost everywhere. A colourless and odourless gas formed from the radioactive decay of radium, it’s not the radon itself that is a health concern, but the radioactive products into which it decays. Radon, being a gas, is simply the vehicle by which members of the uranium decay chain can enter the lungs. Outside the body, radon is not a concern since the alpha particles it emits cannot penetrate the skin.
Beta particles are electrons emitted from the nuclei of many fission products. They can travel a few feet in air but can usually be stopped by clothing or a few centimeters of wood. They are considered hazardous mainly if ingested or inhaled, but can cause radiation damage to the skin if the exposure is large enough.
Neutrons, which are contained in the nucleus of an atom, can be expelled during fission or when alpha particles from radioactive decay interact with nearby nuclei. They interact weakly with matter and are very penetrating—not easy to stop. Neutron radiation typically occurs inside nuclear reactors but water and concrete provide effective shielding. They can also be produced when alpha particles from the decay of uranium react with fluorine nuclei in UF6, for example.
Gamma rays are a form of electromagnetic radiation (like light, radio, and television) that comes from the nucleus of a radioactive atom. They penetrate matter easily and are best stopped by water or thick layers of lead or concrete. Gamma radiation is hazardous to people inside and outside of the body.
How is radiation exposure measured?
When ionizing radiation penetrates a substance, it deposits energy. The energy absorbed by that substance is called a dose. Dose quantities are described in three slightly different ways: absorbed, equivalent, and effective.
The absorbed dose is the amount of energy deposited in a substance. It is measured in a unit called the gray (Gy) and a dose of one gray is equivalent to a unit of energy (joule) deposited in a kilogram of a substance.
However, the biological effect of radiation depends both on the type of radiation (alpha, beta, gamma, etc) as well as the tissue or organ receiving the radiation.
The equivalent dose provides a single measure which accounts for the degree of harm of different types of radiation. To obtain the equivalent dose, the absorbed dose is multiplied by a specified radiation weighting factor (wR). This weighted factor, expressed as the Sievert (Sv), means that an equivalent dose of 1 Gy of alpha radiation would have the same biological effects as 1 Gy of beta radiation.
Different tissues and organs have different radiation sensitivities. For example, bone marrow is much more radiosensitive than muscle or nerve tissue. To obtain an indication of how exposure can affect overall health, the equivalent dose can be multiplied by a factor related to the risk for a particular tissue or organ. This multiplication provides the effective dose absorbed by the body. The unit used to express the effective dose is also the Sievert.
Because doses to workers and the public are so low, most reporting and dose measurements use the terms milliSievert (mSv) or microsievert (μSv) which are 1/1000 and 1/1000000 of a sievert respectively.
How are people exposed to radiation?
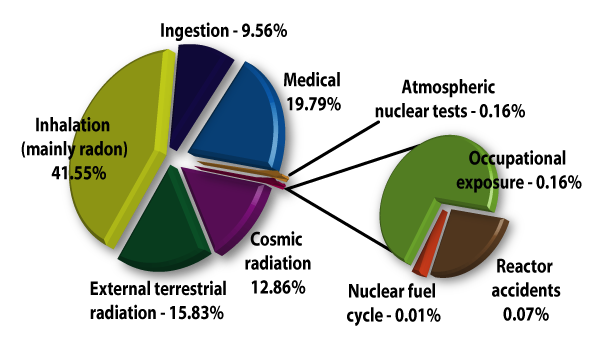
Life everywhere on earth has evolved in an environment containing significant levels of both non-ionizing and ionizing radiation. To a large degree our bodies are adapted to these natural levels of radiation.
Microwave ovens, global positioning systems, cellular telephones, television stations, FM and AM radio, baby monitors, cordless phones, garage-door openers, and ham radios all make use of non-ionizing radiation. The health effects of non-ionizing radiation are negligible.
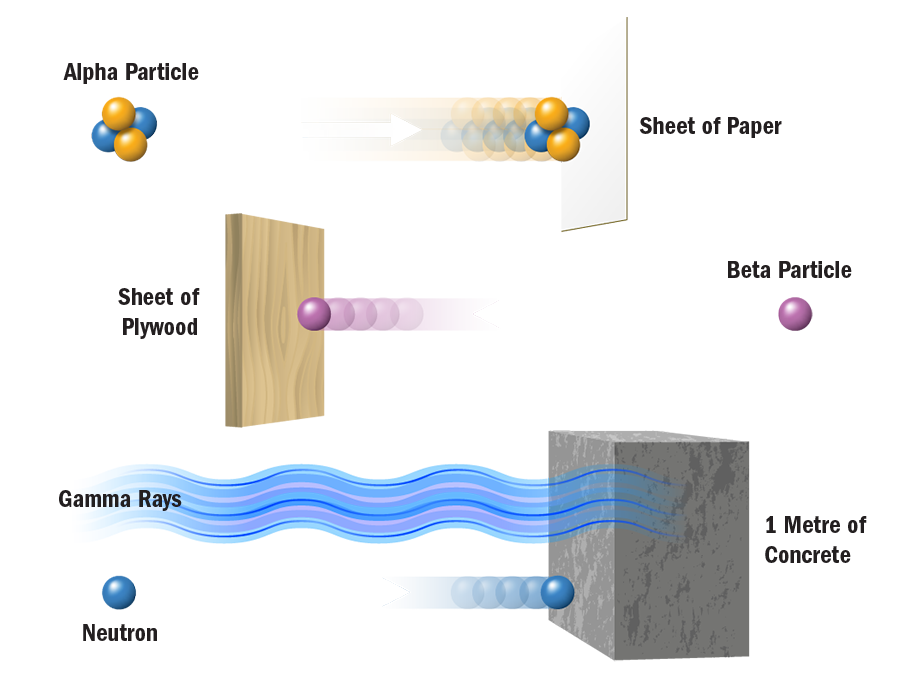
Most natural substances contain some form of radioactive material - soil and rocks, rivers and oceans, food and drinks, and even our own bodies. Radon gas, a natural decay product of radium which occurs in soils everywhere, is a leading source of ionizing radiation. A typical backyard, with dimensions of 10 metres by 10 metres and a soil depth of one metre, contains about 300 grams of uranium. A typical backyard is therefore slightly radioactive.
Cosmic radiation, originating from particles in space that penetrate our atmosphere are also a source of natural radiation that mankind cannot control. People living in higher elevations, for example Denver, CO, naturally receive higher radiation doses from cosmic rays than those living in lower lying areas. Airline personnel and frequent travelers who spend many hours flying at high elevations are also exposed to higher levels of ionizing radiation from cosmic sources.
Almost 80% of all ionizing radiation that people are exposed to occurs naturally.
However radiation is also created artificially to benefit humanity.
- In nuclear medicine, radioisotopes are used for diagnosis, treatment, and research. Radioactive chemical tracers and advanced medical imaging technologies provide highly detailed diagnostic information about a person's anatomy and the functioning of specific organs. In biochemistry and genetics, radionuclides label molecules and allow tracing chemical and physiological processes occurring in living organisms, such as DNA replication or amino acid transport. Radiation treatment is also used to combat many forms of cancer. The medical use of radiological imaging devices (CT scans, X-rays, etc.) contributes almost 20% to the natural amounts of ionizing radiation that we are exposed to.
- In agriculture, radioactive materials are used to develop hardier crops, to control insect pests, and to study such things as plant absorption of fertilizer to optimize the rate of application and avoid unnecessary pollution of soil and the water table.
- In food preservation, radiation is used to stop the sprouting of root crops after harvesting, to kill parasites and pests, and to control the ripening of stored fruit and vegetables.
- Radiation is used to check for flaws when manufacturing jet engines, to toughen rubber in radial tires, to eliminate static in photocopiers and to scan luggage at airports. Radioactive materials are used in industry to verify the quality of steel, in the production of coated paper, in highway construction to test the density of road surfaces, and to test the strength of welds on pipes.
- Radioactive tracers also help analyze pollutants, to study the movement of surface water, and to measure water runoffs from rain and snow, as well as the flow rates of streams and rivers.
- Natural radionuclides are used in geology, archaeology, paleontology and museums to measure ages of rocks, minerals, fossil materials and even authenticate works of art.
- Radioactive materials are also used in consumer products such as smoke detectors to improve their performance.
Radiation from the nuclear industry, including military testing and accidents, accounts for less than 1% of all radiation people are routinely exposed to. In fact, the nuclear fuel cycle accounts for only 0.01%.
What are the effects of radiation?
Our knowledge of the effect of radiation on people is derived primarily from those who have received high doses. The risk associated with very high radiation doses is well established, indicating that high doses of radiation can increase the rate of cancer and genetic damage in the body and can have an immediate effect. This may include reddening of the skin, nausea, or even death in extremely high cases. In fact, it is high doses of targeted radiation that is used in the treatment of cancerous tumours, which kills tumour cells.
We also know that normal exposure such as that received from the environment, normal work activity exposures, exposures to manufactured products containing radioactive materials — like smoke detectors, and medical procedures like X-rays, have no noticeable effects.
But the effects associated with long-term, low level exposures (under about 200 mSv) are less well understood. Because of the large underlying incidence of health issues that may be caused by other factors (such exposure to other known carcinogens, genetics and lifestyles) it is very difficult to scientifically validate the effects of long term low level exposures. Consequently, today’s radiation protection standards assume that any dose of radiation above “normal exposure”, involves a possible risk to human health.
What are safe levels of exposure?
In Canada, the country’s nuclear safety standards are benchmarked against international standards established by a variety of international agencies including:
- International Atomic Energy Agency (IAEA)
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR)
- International Commission on Radiological Protection (ICRP)
The over-riding principle which is applied is that all radiation doses should be kept ‘as low as reasonably achievable’ (ALARA). Canada’s effective dose limits (for man-made sources of radiation) are defined in the CNSC’s Radiation Protection Regulations as follows:
| Person | Period | Effective Dose (mSv) |
|---|---|---|
| 1. Nuclear energy worker, including a pregnant nuclear energy worker | (a) One-year dosimetry period | 50 |
| (b) Five-year dosimetry period | 100 | |
| 2. Pregnant nuclear energy worker | Balance of the pregnancy | 4 |
| 3. A person who is not a nuclear energy worker | One calendar year | 1 |
Nuclear safety regulators in other countries often set similar effective dose limits, basing them at least in part on international standards established the agencies noted above.