The Atom that Changed the World
| Color: | silvery-white |
| Atomic weight: | 238.0289, no stable isotopes |
| State: | solid |
| Melting point: | 1135 ℃, 1408 K |
| Boiling point: | 4130 ℃, 4403 K |
| Electrons: | 92 |
| Protons: | 92 |
| Neutrons in most abundant isotope: | 146 |
| Hardness: | 6.0 mohs |
| Reaction with air: | mild, ⇒ U3O8 |
| Oxide(s): | UO, UO2, UO3, U2O5, U3O8 |

Atomic level properties
Before we talk about uses for uranium, it’s important to understand the unique properties of this remarkable element, beginning at the atomic level.
An atom resembles a miniature solar system. In the centre of the atom is the nucleus around which electrons orbit, like planets moving around the sun. The nucleus, composed of protons and neutrons, contains most of the mass of the atom. Electrons move around the nucleus in relatively large orbits with nothing in between.
Atoms that contain an equal number of protons and electrons are referred to as elements. There are 90 kinds of naturally occurring elements. An atom of uranium is the heaviest found in nature, with 92 protons in its nucleus. It’s even heavier than lead!
Isotopes are variants of a particular chemical element. While all isotopes of a given element share the same number of protons, each differs from the rest by the number of neutrons it has. The sum of the protons and neutrons in the nucleus of an isotope is defined as the mass, therefore each isotope of a given element has a different mass number (ie: U238, U235, etc.)

Of the first 82 elements in the periodic table, 80 have isotopes that are considered stable; they do not change. However, all the elements beyond 82 have unstable isotopes. This means they naturally change (or decay), usually over extremely long periods of time, into different isotopes. As they do so, they release energy in the form of radiation. Uranium’s isotopes are continually changing as they undergo natural radioactive decay.
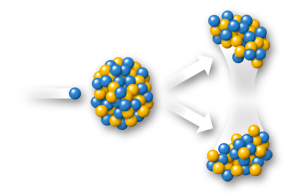
Although science has identified 21 different uranium isotopes, some of them chemically engineered, natural uranium is a mixture of primarily two major isotopes, all with 92 protons, but differing numbers of neutrons. U238 is the most abundant (99.270%), has 146 neutrons and is the most stable natural uranium isotope with a half-life of approximately 4.468 billion years. U238 is not fissile and cannot be used directly as nuclear fuel.
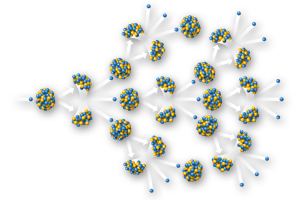
However, it is the isotope U235 (0.725%) with 143 neutrons and a half-life of about 703.8 million years, that has changed life on earth. It is fissile, which means if its nucleus is struck by another neutron, it can release energy by splitting into smaller fragments. If some of the fragments are other neutrons, in turn they can strike other U235 atoms and cause them to split too, creating a nuclear chain reaction.
Uses for uranium
It is this property that gives U235 a starring role in the creation of nuclear energy. When used as nuclear fuel, the most commonly known use for uranium, this isotope is an astonishingly efficient source of energy for generating electricity throughout the world.
According to Einstein's formula, E = mc2 (Energy equals mass multiplied by the square velocity of light - c2) very small amounts of mass may be converted into very large amounts of energy.

This means that one tiny pellet of uranium that weighs about 0.24 ounces can generate as much energy as 3 barrels of oil, 17,000 cubic feet of natural gas, or 2,000 pounds of coal.