Concentrating U235
The most common type of nuclear power generating reactor, light water reactors, require concentrations of between 3% -5% of the fissile isotope U235. Natural uranium contains only 0.7% U235. Therefore, most commercial nuclear power reactors require enriched uranium as their fuel.
Because the isotope U238 is slightly heavier than U235, the two can be separated. This is accomplished through physical processes called enrichment that concentrates the U235 isotope.
There are two enrichment methods used at a commercial scale today; gaseous diffusion and centrifuge. Laser enrichment, a promising new technology, is being developed in the U.S.
Gaseous diffusion
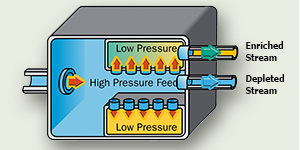
Once heated to a gaseous state, the UF6 is fed into the plant's cascades to be enriched. The gas is forced through membranes with microscopic openings. Because the U235. is lighter, it moves through the barriers more easily.
As the gas moves, the two isotopes are separated, increasing the U235. concentration to the desired level and decreasing the concentration of U238..
Gaseous diffusion requires large amounts of electricity to achieve the enrichment levels required for reactor use. As a result, it has been largely phased out.
Only two countries have used this process on any significant scale over the past few years. The Georges Besse 1 gaseous diffusion plant in France shut down permanently in mid-2012. The only other major diffusion plant that is still operating, located in Paducah, KY, is scheduled to close in mid-2013.
Centrifuge
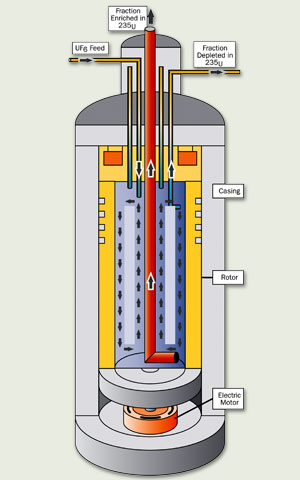
In this enrichment process, gaseous UF6 is pumped into a centrifuge (a cylindrical container that spins the UF6 at high speeds). Centrifugal force pushes the heavier U238 particles to the outside of the centrifuge. The lighter U235 particles concentrate at the centre.
A number of centrifuges are connected together in an arrangement known as a cascade. As the UF6 passes through successive cascades, the concentration of U235 gradually increases.
Enrichment using centrifuge technology requires little energy, giving this method a significant cost advantage. Centrifuge enrichment requires only about 2% of the energy needed for gaseous diffusion.
Large commercial centrifuge enrichment plants are in operation in France, Germany, Netherlands, the U.K., the U.S.A., and Russia, with smaller plants elsewhere.
After enrichment
Enrichment produces two streams of UF6 one with elevated concentrations of U235 to be used as reactor fuel and the left-over tailings with low levels of U235 and higher levels of U238.
Before the enriched UF6 can be used in light water reactors, it is chemically transformed back into UO2 powder which can then be processed into pellets in the manufacturing process.
The tailings are collected as a by-product commonly known as DUF6 (an acronym for depleted UF6). It is often referred to as "depleted tails" or "depleted uranium” because it contains low amounts of U235.
Because 99.3% of natural uranium consists of U238 isotopes, large quantities of DUF6 are produced during the enrichment process. These tailings are stored and managed in secure, air-tight cylinders on the enrichment facilities’ sites.
DUF6 is mildly radioactive and is safely stored within the containers. However, the chemical hazards from the compound, in particular fluorine components, need to be managed. It is corrosive and thorough, ongoing maintenance of storage cylinders is required.

Therefore, it's preferable from both an environmental and economic standpoint to deconvert DUF6 back into separate fluorine compounds and uranium (as either UO2 or U3O8). UO2 and U3O8 are both chemically stable and, when produced from enrichment tails, emit even lower levels of radiation than natural uranium. There is commercial value for fluorine-based products and some value in depleted uranium metal for use as counterweights, shielding materials etc. Depleted uranium in oxide form can be safely disposed as low-level radiological waste.
Four deconversion plants are in operation today – one in France, one in Siberia and two in the US, with a fifth under construction in the New Mexico.
Enrichment and non-proliferation
Nuclear weapons require enriched uranium at levels far beyond those required for nuclear power generation. For comparison purposes, natural uranium contains 0.7% U235. Enriched uranium for nuclear power reactors contains 3%-5% U235 while weapons-grade, highly enriched uranium (HEU) contains at least 90% U235. Because the process to create weapons-grade uranium is fundamentally the same, from a non-proliferation standpoint, uranium enrichment is a sensitive technology.
Enrichment processes are subject to rigorous international controls on sharing of technology, precise inventory accounting, reporting and in-depth security measures; all designed to ensure nuclear materials are used only for peaceful purposes.